Articles & Whitepapers

Nitrosamine Navigation
Download Now

Responding to Consumer Complaints
Download Now

Outsourcing Stability Testing
Download Now

Governance for Outsourced Facilities Management
Download Now

Introduction to Phase Appropriate cGMP
Download Now
Considerations for Analytical Method Validation Lifecycle Controls
Download Now

Effective Post Market Supplier Strategy for Combination Products
Download Now

KASA to Support Generic Drug Review
Download Now

Nitrosamines
Download Now

The Real Cost Of Poor Data Integrity In Pharmaceutical Manufacturing.
Download Now

Whitepaper: What you don’t know CAN hurt you.
Download Now

Don’t Wait! Keeping Up With Medical Device Compliance.
Download Now

Navigating Through The Cloud(s) In Life Sciences.
Download Now

From Big Data to Cybersecurity
Download Now

Avoid Data Integrity Disasters
Download Now

Supply Chain Risk Mitigation
Download Now

Avoid Sponsor-Supplier Pitfalls
Download Now

Warning Letters
Download Now

Data Integrity Guidance
Download Now

ANDA Refuse-to-Receive Pitfalls
Download Now

Elemental Impunity
Download Now

FDA’s Information Transparency
Download Now

Reporting CPPS to FDA
Download Now
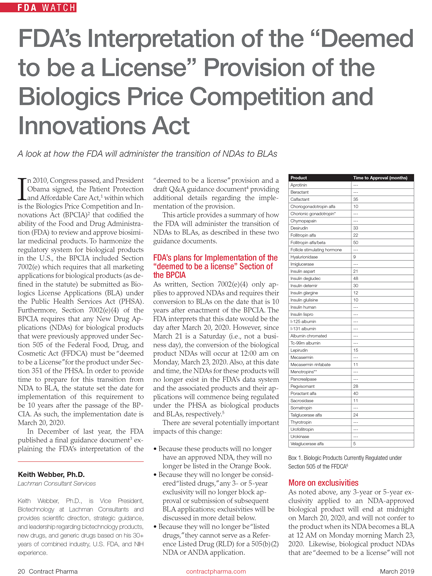
Deemed to be a License
Download Now

Leverage FDA Data
Download Now

Awareness and Precision
Download Now

Requirements for SaMD
Download Now

AWS Products in GxP Systems
Download Now
ADVANCE CONFIDENTLY
Contact Us Today








